Group Sabine Müller
 |
Molecular Mechanisms of Cellular Morphogenesis
Control of cell morphology is a fundamental property of life, essential to the form and function of all organisms. Polarized cell division and cell expansion are the key determinants of cellular morphogenesis and constitute prerequisites of three-dimensional organ development, tissue functionality, and adaptations to changing environmental conditions on multiple scales. Cell shape is governed directly or indirectly by an internal scaffolding structure called the cytoskeleton. The dynamically reorganizing cytoskeleton is a major driver of cell division and growth, fixing division planes, segregating chromosomes, and directing targeted vesicle trafficking during cell division and subsequent anisotropic and multipolar growth. My lab uses the root meristem and the leave epidermis of the model plant Arabidopsis thaliana as traceable experimental systems to investigate molecular mechanisms involved in cellular morphogenesis. We use a synergistic combination of molecular genetics and live-imaging to investigate cell-shaping processes (Figure 1). This approach enabled the discovery of novel components in mechanisms underlying the complex and multi-scale processes linking cytoskeletal proteins and signaling complexes to cell shape determination and consequently plant development.
 |
 |
||
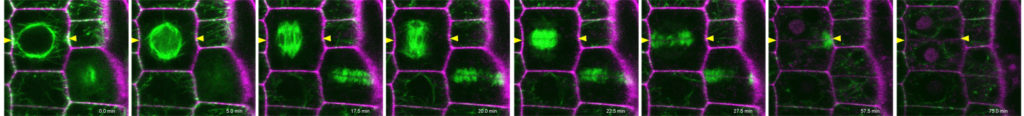
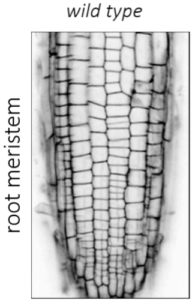
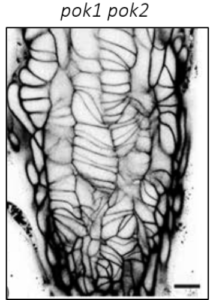
| Figure 1: Arabidopsis root meristem cellular organization and cell division in wild type and kinesin 12 pok1pok2 double mutant. | |||
Division plane maintenance and the roles of kinesin-12 motor proteins
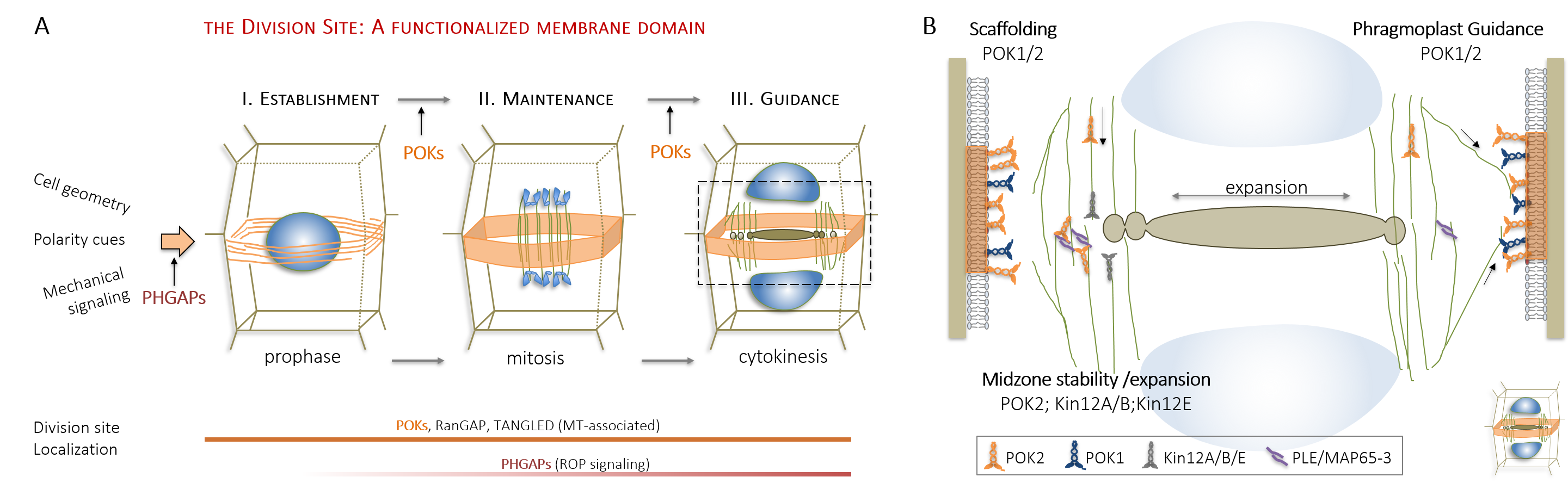
Research in my lab has significantly progressed the understanding of division plane orientation, a question engaging scientists since the 19th century. Using advanced imaging techniques, we characterized the spatio-temporal dynamics of the Arabidopsis kinesin-12 class motor proteins PHRAGMPOLAST ORIENTING KINESIN (POK) 1 during cell division . Combining imaging with molecular genetics, our research proofed the essential role of kinesin POK1 and its close relative POK2 as core components and master-regulators of the division site, a polarized plasma membrane region that predicts and preserves the division plane throughout cell division (Figure 2A, Lipka et al., 2014, Livanos and Müller, 2019). Analysis of spatio-temporal localization patterns, biochemical, and genetic interaction assays support POK kinesins function as a scaffold that maintains some division site resident proteins. Besides, we revealed their importance for phragmoplast guidance on a multi-scale level and uncovered the contribution of POK2 to phragmoplast stability and expansion via two separate binding domains with differential binding specificities for members of the microtubule cross-linker MAP65 (Figure 2B, Herrmann et al., 2018).
 |
|
| Figure 2: Division site establishement and Phragmoplast Guidance. (A) Division site resident proteins during cell division. (B) Kinesin-12 functions during cytokinesis. |
Future Directions:
Molecular mechanisms of Phragmoplast guidance
Characterization of the POK division site association.
Evolution of kinesin-12 dependent phragmoplast guidance and division zone maintenance
Extending the cortical division zone interactome
ROP-mediated cell polarity in division plane selection of plant cells
Previous and Current Research & Impact: In our previous work, we characterized the novel interactors of POK1/2, designated PHGAPs . These proteins belong to a family of GTPase activating proteins (GAP) that deactivate the cell polarity signaling molecules Rho of plants (ROP). PHGAPs enrich at the division site in a POK-dependent manner only after prophase (Figure 2A, Stöckle et al., 2016). However, cytoskeleton analysis in the phgap1 phgap2 root meristem revealed a failure in division plane selection during prophase that also affected POK localization. In brief, this work suggested that cell polarity cues guide division plane selection not only in asymmetrically but also in symmetrically dividing cells of the root meristem.
(1) Regulation of PHGAP division site localization.
(2) Establishment of the actin depleted zone.
Evolution of Kinesin-12 functions
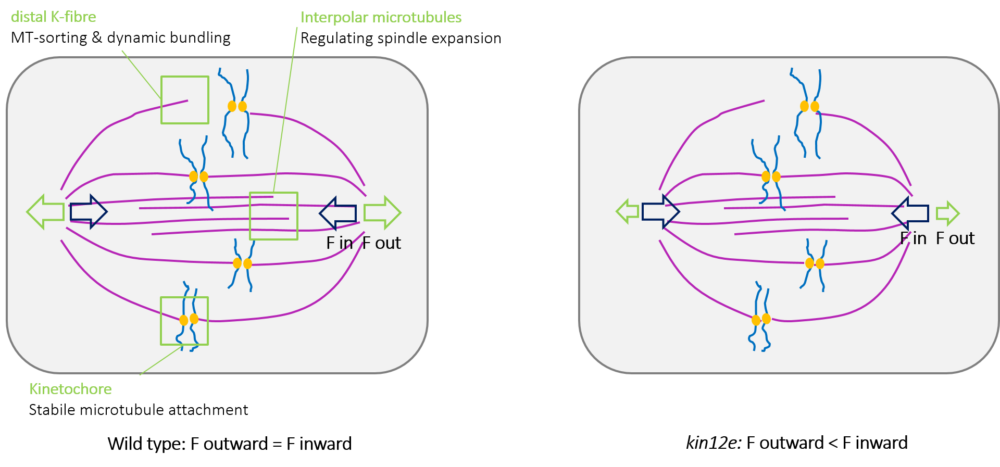
Previous Research & Impact: The bipolar mitotic spindle is a highly conserved structure among eukaryotes that mediates chromosome alignment and segregation. Spindle assembly and size control are facilitated by force-generating microtubule-dependent kinesins. Arabidopsis KINESIN-12E functions in spindle assembly. In kinesin-12e mutants, a delay in spindle assembly is accompanied by the reduction of spindle size (Figure 3), demonstrating that KINESIN-12E contributes to mitotic spindle architecture. Changes in the kinetochore and in prophase and metaphase spindle dynamics occur in the absence of KINESIN-12E, suggest it might play an evolutionarily conserved role during spindle formation similar to its spindlelocalized animal kinesin-12 orthologs.
 |
|
| Figure 3: Kinesin-12E regulates spindle size. |
Cell polarity establishment in pavement cell development
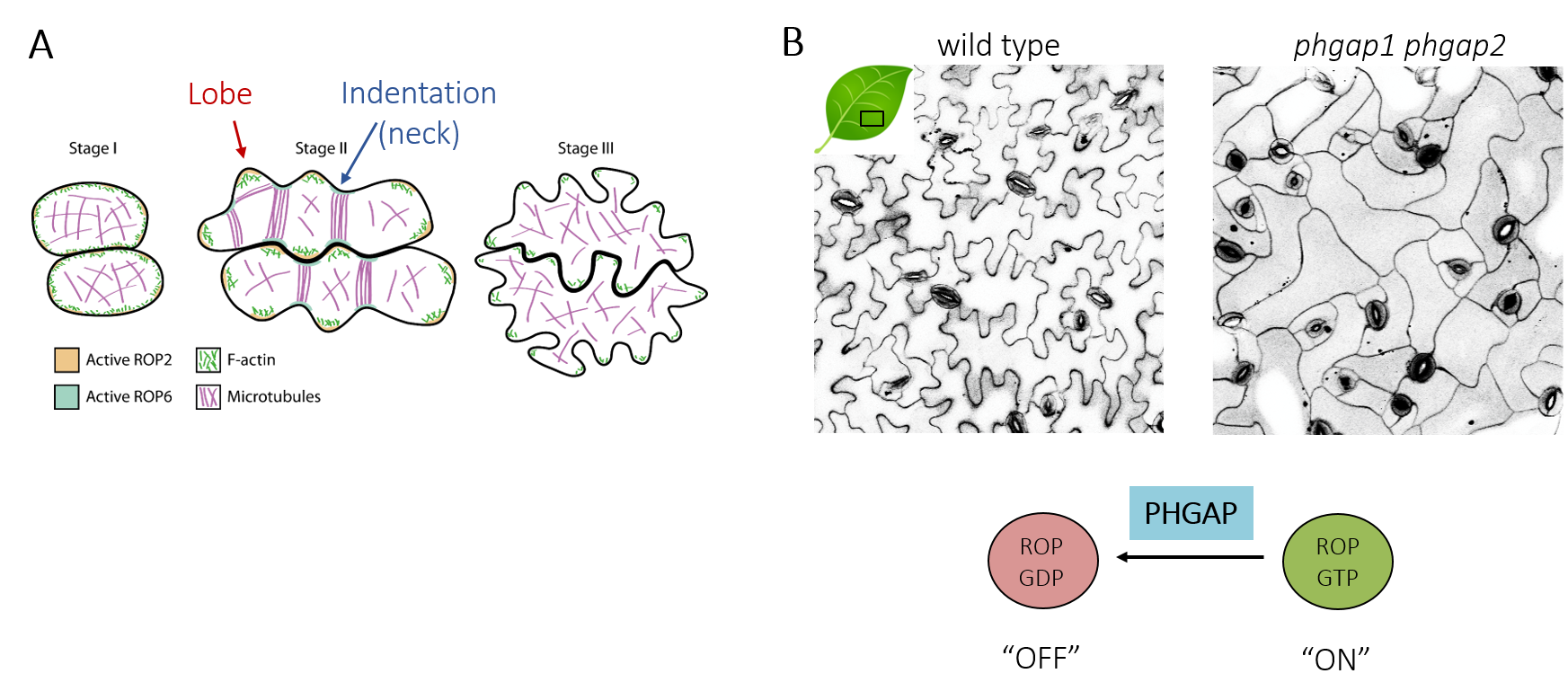
Ongoing & future research: In addition to cell division, PHGAPs are involved in the establishment of cell polarity in multi-polar pavement cells of the leaf epidermis. These cells have an intricate interlocked shape (Figure 4A) that is thought to reduce mechanical stress across the tissue . The development of pavement cells involves iterative feedback between microtubules that spatially instruct cell wall deposition to resist turgor pressure and anisotropic cell expansion that in turn feeds back onto microtubule distribution. The mechanical stress arising during this recursive process further modulates cellular behavior.
PHGAP functions during pavement cell development. Pavement cell shape complexity is lost in phgap mutants, placing its function within the ROP signaling pathway (Figure 4B). Further characterization employing advanced imaging and will elucidate PHGAP dynamics to determine the requirements for PHGAP polarization for cellular morphogenesis leveraging mutants with altered pavement cell morphology. This work will clarify how PHGAP activities fine-tune multipolar shape formation.
 |
|
| Figure 4. Pavement cell development (modified from Craddock et al., 2012). (A) Correlation of ROP activities and cytoskeletal organization instruct shape formation. (B) Shape of Leave epidermis pavement cells and ROP dependent cell polarity signaling. |
Team
Dr. Pantelis Livanos
Choy Kriechbaum (PhD student)
Jennifer Schuster (TA)