Group Benedikt Kost
Mechanism and regulation of polar plant cell growth
A. Research aim and experimental models
Migration and morphological plasticity of cells within developing plant organs are strongly limited by the presence of cell walls. Directional (polar) cell growth therefore plays a key role in the morphogenesis of cells and organs particularly in plants. Vegetative pollen tube cells and apical cells of moss protonema rapidly elongate in a strictly polar manner by tip growth, a process that requires cytoskeleton dependent massive secretion of cell wall material specifically at the extreme tip.
We are employing tobacco pollen tubes and Physcomitrella patens protonema as model systems to investigate cellular and regulatory mechanisms underlying polarity and directional cell expanison in plants.
B. Control of pollen tube growth by Rac/Rop signaling
Small GTPases of the Rac/Rop family accumulate at the plasma membrane (PM) specifically at the tip of pollen tubes, and control the polar growth of these cells (Figure 1; Kost et al. 1999). Pollen tube Rac/Rop GTPases interact with a lipid kinase activity responsible for the accumulation of the signaling lipid phosphatidylinositol 4,5- bisphosphat (PI 4,5-P2) in the PM at the pollen tube tip (Figure 1; Kost et al. 1999).
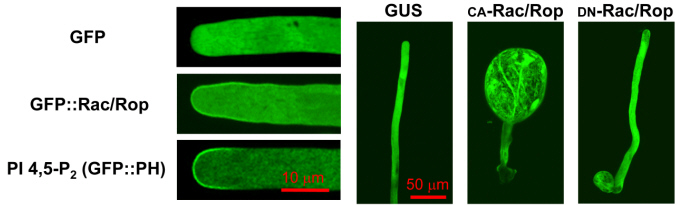
GFP fused to Rac/Rop, or to the PH domain of Rn-PLCd1 (specific PI 4,5-P2 marker), accumulates at the PM specifically at the pollen tube tip. Expression of constitutively active (CA) Rac/Rop depolarizes pollen tube growth, whereas dominant negative (DN) Rac/Rop inhibits this process (Kost et al. 1999).
Pollen tube Rac/Rop GTPases belong to the large Rho family of small GTPases, which has been well characterized in non-plant systems. Rho GTPases typically stimulate multiple signal transduction pathways and cellular processes when bound to GTP, whereas their signaling function is inactive in the GDP bound conformation. The ratio at which GTP (active) and GDP (inactive) bound forms of Rho GTPases are present in living cells is controlled by a group of conserved regulatory proteins (Figure 2). We are contributing to an improved understanding of how functional interactions between regulatory proteins and other factors maintain the polarization of Rac/Rop signalling at the tip of pollen tubes (Klahre et al. 2006, Helling et al. 2006, Klahre and Kost 2006). In addition, our work enhances current knowledge of the signaling network stimulated by pollen tube Rac/Rop activity (Kost et al. 1999; Kost 2008; Yalovsky 2008). Clearly, Rac/Rop signaling relies on plant specific upstream regulatory mechanisms and downstream signal transduction pathways.
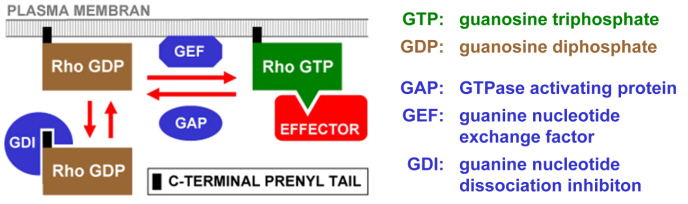
Posttranslational prenylation is responsible for the association of Rho GTPases with the PM. In the active GTP bound conformation, Rho GTPases typically interact with multiple effectors to simulate downstream signaling. GAPs increase the GTPase activity of Rho GTPases and inhibit signaling that depends on these proteins. GEFs promote nucleotide exchange and stimulate Rho dependent signaling cascades. GDIs transfer GDP bound Rho GTPases from the PM to the cytoplasm.
C. Control of moss tip growth by Rac/Rop signaling
The moss Physcomitrella patens is the only currently available model plant that integrates transgenes by homologous recombination with a frequency high enough to allow effective gene knock-out and/or gene replacement. Based on this feature and on the availability of a well accessible genome sequence, P. patens is highly suitable as a complementary experimental system to study polar plant cell growth.
An extensive analysis we have performed established that the P. patens genome encodes homologs of all major Rac/Rop signaling proteins known from higher plants, including 4 nearly identical Rac/Rop GTPases (PpRop1-4; Eklund et al., 2010; Figure 3). One of these Rac/Rop homologs (Pp-Rop1) fused to GFP accumulates at the PM specifically at the tip of apical cells of P. patens protonema, which elongate by tip growth (Figure 3). The functions of all Pp-Rop proteins and of other P. patens Rac/Rop signaling proteins are now further investigated.
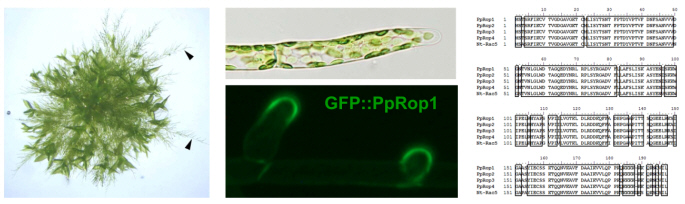
Branching protonema (left, arrow heads) extend from a P. patens colony growing on the surface of solid culture medium. The apical cell at the end of each protonemal filament (center, top) elongates by tip growth. The P. patens Rac/Rop homolog Pp-Rop1 specifically accumulates at the tip of growing apical cells (center, bottom). The amino acid sequences of Pp-Rop1, of the three other P. patens Rac/Rop homologs and of the tobacco pollen tube Rac/Rop GTPase Nt-Rac5 are highly similar (right).
D. Key goals of ongoing research
- elucidation of regulatory mechanisms that polarize Rac/Rop signaling in tip growing cells
- characterization of signaling cascades stimulated by Rac/Rop activity in these cells
- identification of cellular processes controlled by these signaling cascades.
We are employing cell biological, molecular biological, biochemical and genetic tools along with mathematical modeling to work towards these goals.
Team
Dr. Maria Ntefidou
Joline Blaß (PhD student)
Carolin Fritz (PhD student)
Johanna Knab (PhD student)
Sylwia Schulmeister (TA)
Martin Schuster (TA)
Hildegard Voll (TA)
Publications
 |
 |
 |